Pediatric immunotherapy (Lymphoma) – Dr. Francesco Ceppi (CHUV)
Immunotherapy for relapsed-refractory pediatric and young adult B-cell precursor acute lymphoblastic leukemia: CAR-T cell clinical trial development
This “allocated fund in pediatric oncology” was awarded to Dr. Francesco Ceppi in September 2019.
B-cell precursor acute lymphoblastic leukemia (BCP-ALL) is the most common childhood malignancy. It can be successfully treated and presents a good survival rate. Most therapy failures are still due to relapses, which occur in approximately 15–20% of all patients. Therefore, novel therapies are needed for children with relapsed/refractory BCP-ALL.
Immunotherapeutic approaches have recently come to the forefront in BCP-ALL therapy.
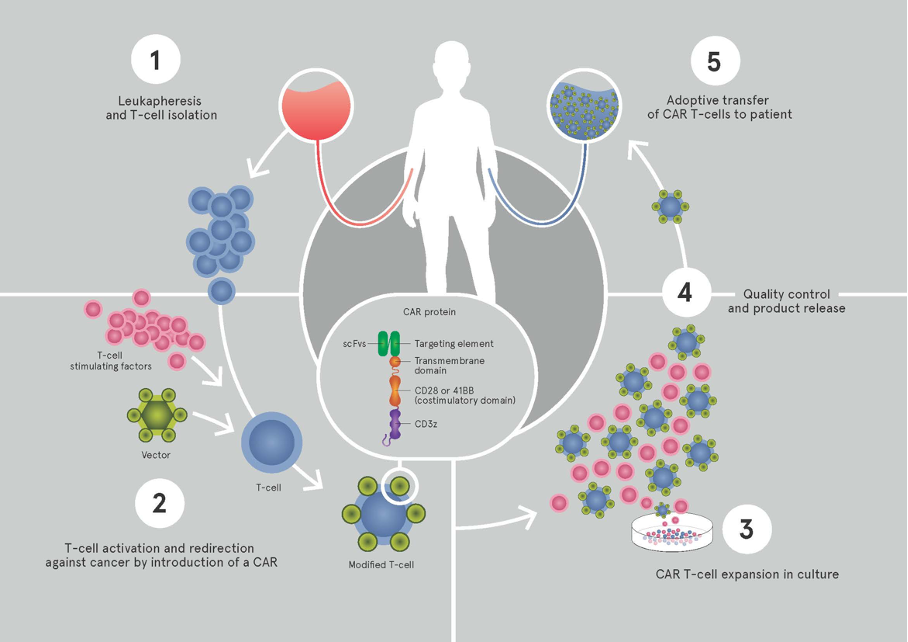
Infusion of autologous T lymphocytes (T cells) engineered to express a chimeric antigen receptor (CAR) with CD19 specificity has been associated with consistent responses in early-phase clinical trials in the USA for relapsed/refractory BCP-ALL (complete remission rates of 70-90%).
Although the initial remission rates were very impressive across the trials, as noted above, approximately 50% of patients relapsed within a year. Taken together, approximately half of the relapses are due to lack of persistence of the CAR T cells, suggesting that some minimum time of persistence is required for sustained remissions. One explanation for short persistence is an immune-mediated rejection of the CAR construct, typically aimed at the murine-based scFv domain. Therefore, more recent efforts have focused on the use of humanized or fully human CAR constructs. Using a humanized anti-CD19 CAR construct is hypothesized to improve persistence and therefore outcomes.
The principal aim of the proposed research will be to develop and open a pediatric clinical trial using a fully human CD19-targeting CAR for relapsed/refractory CD19+ leukemia and lymphomas in Switzerland (and Europe). The work will be performed in an academical setting, exploiting the immunotherapy platform of the Oncology Department at CHUV (Prof. G. Coukos and Prof. L. Kandalaft), in collaboration with the Seattle Children’s Hospital. The study will examine the efficacy of administering a T cell product derived from autologous peripheral blood mononuclear cells that have been genetically modified using a self-inactivating (SIN) lentiviral vector to express a fully human CD19-targeting CAR. Subjects will receive a single dose of humanized CD19 CAR T cells. CD4 and CD8 T cell subsets will be isolated from an apheresis product obtained from the research subject. The isolated cells will be stimulated with anti-CD3 x anti-CD28 beads and transduced with a SIN lentiviral vector that directs the co-expression of the fully human CD19. Modified cells will be propagated to numbers suitable for clinical use using recombinant human cytokines. The product will be cryopreserved and release testing will be performed. Following lymphodepleting chemotherapy, the T cell product will be administered to the subject.