Benedetta Fiordi, PhD in the laboratory of Prof. Camilla Jandus (UNIGE) – (Acute myeloid leukemia)
AML blasts shed TREM2 to inhibit ILC function
Benedetta Fiordi was awarded this “PhD scholarship” in March 2023 for 3 years. She is performing her research in Prof. Camilla Jandus’ lab at the University of Geneva.
The immune system and the peripheral nervous system are present throughout the body and cooperate to ensure vital organs maintain tissue homeostasis and the overall health of the host. However, it is still poorly understood how this cooperation is modified or lost in cancer, in particular in leukemia, and more thorough investigation is needed to identify targetable determinants able to restore the correct crosstalk. Leukemias define a group of blood cancers originating in the bone marrow (BM), a highly innervated tissue.
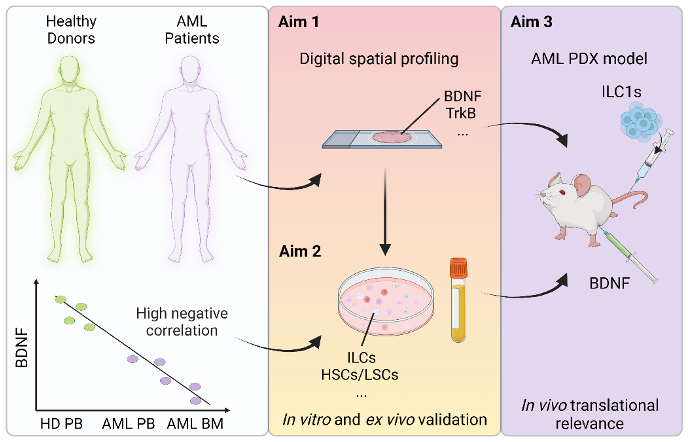
In this project, I am focusing on acute myeloid leukemia (AML), a disease that urgently requires a more complete understanding, the overall survival rate being below 20% due to drug resistance and disease relapse. The main cause of treatment failure in AML are leukemic stem cells (LSC) that are resistant to chemotherapy, that survive and give rise to other leukemic clones that proliferate and engraft in the patients. LSCs reside in the BM and are sustained by cells and soluble factors, including neural-derived factors, that constitute the BM microenvironment, also called the BM niche. Previously published findings from the host laboratory show that innate lymphoid cells (ILC), a recently described family of immune cells involved in tissue homeostasis, inflammatory diseases and cancer, are present but functionally dysregulated in the BM of AML patients at disease onset and are only partially restored after chemotherapy. However, how ILCs interact with malignant cells, including LSCs, and whether this interaction is modulated by neurotrophic factors is yet to be explored.
The innate lymphoid cell family comprises two major groups: the helper ILC (hILC) and the natural killer (NK) cells. hILC can be divided into three subtypes (ILC1, ILC2 and ILCP), while NK includes NK1/NK3 and NK2 cells. Interestingly, ILC1 and NK share many features: they can be activated by similar cytokines and they both secrete IFN-gamma and TNF-alpha. My PhD project hypothesis is that two factors, the brain-derived neurotrophic factor (BDNF) and sTREM2, could be critically involved in impairing the anti-leukemic ILC functions in AML. I have obtained promising data,primarily on sTREM2, and will focus in this project on the role of sTREM2 on ILC in AML, with a secondary focus on the role of BDNF. The aim is to understand how the loss of these factors affects ILC anti-tumoral functions, promotes LSC survival and/or modulates their interactions.
Overall, I expect to shed light on a new layer of tumor immunity, i.e., the neuro-immune circuit in leukemia, a potential key contributor to disease progression and response to therapy.
Schematic representation of the proposed project