The role of natural killer cells in cutaneous T cell lymphoma: pathophysiological mechanisms and clinical implications
Christoph Iselin was awarded this “MD-PhD scholarship” in May 2023 for 3 years. He is performing his research in Prof. Emmanuella Guenova’s lab at the University of Lausanne.
Cutaneous T cell lymphoma (CTCL) is a group of rare but potentially lethal non-Hodgkin’s lymphomas, originating from malignant CD4+ T cells. The patient’s immune system is deemed an important determining factor of disease progression, and immunomodulatory therapies reliably improve outcomes. Therefore, understanding the effect of CTCL on the immune system and its cells is imperative for improving therapeutic options.
CTCL immunomodulatory effects lead to immune cell exhaustion, explaining the so far disappointing results of T- and dendritic cell-dependent therapies when compared to other types of cancer. However, there is recent evidence that natural killer (NK) cells account for the response of immunomodulatory treatment in CTCL.
The overarching goal of this project is to reverse the CTCL-impaired NK cell activity by targeting the factors which negatively regulate NK cell function. The first step will be to phenotype the NK cells in the tissue microenvironment (TME) of CTCL through computational, single-cell sequencing analysis. I will examine this comprehensive dataset for factors and pathways that specifically alter NK cells in CTCL by comparing it with that of the NK cells in healthy skin, inflammatory skin diseases and solid skin tumors.
All discovered candidate molecules will then be individually validated by reverse transcription PCR (RT-PCR), fluorescence-activated cell sorting (FACS) and protein immunoblotting in NK cells from CTCL patients and individuals suffering from the aforementioned other diseases as controls. The number and state of activity of NK cells will be compared to the existing patient-specific clinical data to evaluate the impact of disease stage and progression. I will then test the reversibility of the suppressive effect of promising candidate molecules on NK cell activity in vitro and develop a combinatorial therapy plan to treat CTCL in a syngeneic T cell lymphoma model. In this way, I will test in vivo whether the target molecules discovered in steps 1 and 2 can be used to rescue the anti-tumor potential of NK cells and improve the outcome of CTCL.
If successful, this project will advance our understanding of the role of NK cells in CTCL pathology and treatment, as well as in inflammatory skin diseases and solid skin tumors. The factors and pathways initially identified through computational analysis, experimentally validated in vitro as reversible and found to be effective in vivo can be the basis for further studies to test their clinical applicability.
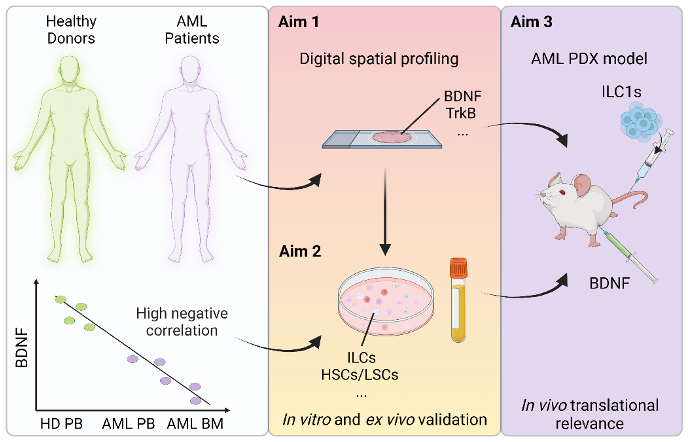
AML blasts shed TREM2 to inhibit ILC function
Benedetta Fiordi was awarded this “PhD scholarship” in March 2023 for 3 years. She is performing her research in Prof. Camilla Jandus’ lab at the University of Geneva.
The immune system and the peripheral nervous system are present throughout the body and cooperate to ensure vital organs maintain tissue homeostasis and the overall health of the host. However, it is still poorly understood how this cooperation is modified or lost in cancer, in particular in leukemia, and more thorough investigation is needed to identify targetable determinants able to restore the correct crosstalk. Leukemias define a group of blood cancers originating in the bone marrow (BM), a highly innervated tissue.
In this project, I am focusing on acute myeloid leukemia (AML), a disease that urgently requires a more complete understanding, the overall survival rate being below 20% due to drug resistance and disease relapse. The main cause of treatment failure in AML are leukemic stem cells (LSC) that are resistant to chemotherapy, that survive and give rise to other leukemic clones that proliferate and engraft in the patients. LSCs reside in the BM and are sustained by cells and soluble factors, including neural-derived factors, that constitute the BM microenvironment, also called the BM niche. Previously published findings from the host laboratory show that innate lymphoid cells (ILC), a recently described family of immune cells involved in tissue homeostasis, inflammatory diseases and cancer, are present but functionally dysregulated in the BM of AML patients at disease onset and are only partially restored after chemotherapy. However, how ILCs interact with malignant cells, including LSCs, and whether this interaction is modulated by neurotrophic factors is yet to be explored.
The innate lymphoid cell family comprises two major groups: the helper ILC (hILC) and the natural killer (NK) cells. hILC can be divided into three subtypes (ILC1, ILC2 and ILCP), while NK includes NK1/NK3 and NK2 cells. Interestingly, ILC1 and NK share many features: they can be activated by similar cytokines and they both secrete IFN-gamma and TNF-alpha. My PhD project hypothesis is that two factors, the brain-derived neurotrophic factor (BDNF) and sTREM2, could be critically involved in impairing the anti-leukemic ILC functions in AML. I have obtained promising data,primarily on sTREM2, and will focus in this project on the role of sTREM2 on ILC in AML, with a secondary focus on the role of BDNF. The aim is to understand how the loss of these factors affects ILC anti-tumoral functions, promotes LSC survival and/or modulates their interactions.
Overall, I expect to shed light on a new layer of tumor immunity, i.e., the neuro-immune circuit in leukemia, a potential key contributor to disease progression and response to therapy.
Schematic representation of the proposed project
Modeling and investigating the tumor microenvironment of non-small cell lung cancer brain metastasis
This « ISREC grant for translational oncology » was awarded to Benoît Duc in November 2021. Benoît Duc works in the group of Prof. Johanna Joyce at the Ludwig Institute for Cancer Research, University of Lausanne.
Lung cancer, including the most common type, non-small cell lung cancer, is the leading cause of cancer-related deaths worldwide. Metastasis represents the final stage of lung cancer progression, when cancer cells have successfully spread to a new organ and colonized it. Critically, 20-40% of lung cancer patients develop brain metastasis over the course of the disease progression. We urgently need to devise novel therapies for lung cancer brain metastasis because, first, more than 50% of the patients die in the year following diagnosis, and second, these metastases cause deaths in patients in which the disease in the lung and other sites of metastasis is under control.
However, the lack of animal models that faithfully represent human lung cancer brain metastasis impedes our current understanding of the underlying molecular mechanisms. Yet this information is urgently required for the identification of the unique vulnerabilities of these mechanisms for future therapeutic targeting.
In this project, we will take advantage of our collaborations with pathologists, surgeons and scientists within the Swiss Cancer Center Léman and overseas, to reveal novel therapeutic combinations that target the non-cancerous cells in the tumors (the tumor microenvironment), including the immune cells. We will generate a first of its kind mouse model of lung cancer that recapitulates all the steps of lung cancer brain metastasis. Since these mice will have an intact immune system (which is not the case in many current models), we will be able to determine how the body’s defenses react to lung cancer metastasis in the brain, and identify therapies that can boost this response.
In parallel, we will leverage novel technologies that describe the cellular interactions that determine how the non-cancerous cells react to the cancer cells and where these interactions occur in the tumor. By performing these analyses in patient samples and in our animal model, we aim to identify yet unappreciated vulnerabilities in the non-cancerous cells of lung cancer brain metastasis, which we will target in our novel mouse model. We hope that this will ultimately lead to a personalized management of non-small cell lung cancer brain metastasis, whereby rationally designed treatments will target distinct molecular subtypes.

