Expanding the knowledge on the potential of cancer vaccines

Lung cancer is, to this day, the leading cause of cancer-associated deaths worldwide. There is a dire need for the development of more effective therapies, as lung tumors often become resistant to both conventional and targeted therapies, such as immunotherapies. This collaboration will work on advancing a promising cancer therapy referred to as dendritic cell (DC) vaccines.
DCs play a fundamental role in orchestrating the functions of our immune system. They present antigens on their surface that are recognized by other players in the immune response. Their biological role has long been exploited to develop DC vaccines for patients with cancer. The goal of a DC vaccine is to make the patient’s own immune system recognize and eliminate the cancer cells. More specifically, immature DCs are isolated from a patient with cancer and then exposed to tumor-associated antigens. After reaching full maturity, the cells are reintroduced in the patient to trigger an anti-tumor response. However, this traditional approach has several limitations and has yielded mixed clinical results.
This TANDEM project aims to advance the design of DC vaccines for lung cancer therapy. It exploits a novel type of DC, which is engineered in the laboratory to improve its ability to present tumor antigens to the immune system. This work is poised to improve the therapeutic potential of DC vaccines and will hopefully provide a new treatment strategy for lung cancer patients.
Employing new visualization technologies will further the understanding of CAR-T cell therapy

The immune system plays a crucial role in inhibiting tumor growth. Adoptive cell therapy is a kind of immune therapy, where the cells of the patient’s own immune system are extracted, reprogrammed separately, and introduced back into the body to combat tumors in a very targeted manner.
More specifically, an adoptive cell therapy that uses chimeric antigen receptor T cells (CAR-T) has been transformative for people suffering from selected hematological malignancies (cancers that begin in the blood forming tissue) that are prone to relapse or are refractory. CAR-T therapies are being expanded to treat solid tumors as well. Unfortunately, there are significant toxicities associated with CAR-T cell therapy, especially since preclinical tools to evaluate CAR efficiency and safety can be inaccurate, time-consuming, and expensive.
The aim of this TANDEM project is to increase the precision and safety of engineered T cells by implementing new microscopy technologies to dissect and examine the interaction between the CAR-T cell and the tumor cell. They aim to make these technologies easy to implement so that they can routinely be used in a clinical setting. They will in turn provide insight on whether the therapy will be effective or generate toxicity. If successful, this new imaging tool will be used to improve both the patient’s prognosis and the patient’s overall quality of life during CAR-T treatment of hematological malignancies.
Understanding disease progression in lung cancer

Lung adenocarcinoma (LUAD) represents 40% of all lung cancers, which makes it one of the most common lung cancers. Previous studies have used histopathology (the study of changes in tissues caused by disease) to diagnose and study diseased lung tissue at the microscopic level. This classic approach identified changes in cell morphology and growth patterns that accompany disease progression. The overarching aim of the current collaboration is to use new techniques to predict more accurately how the cancer will progress and whether or not it will react to treatment.
Disease progression is driven by the plasticity of cell identity and a coincident reshaping of the tumor environment, such that reprogramming is maintained. Based on histopathological analysis, there are four recurrent tumor progression patterns that reflect both tumor aggressivity and patient survival prognosis. The milestones are readily identified and provide a lot of information on disease progression and tumor heterogeneity, both within one patient and among multiple patients:
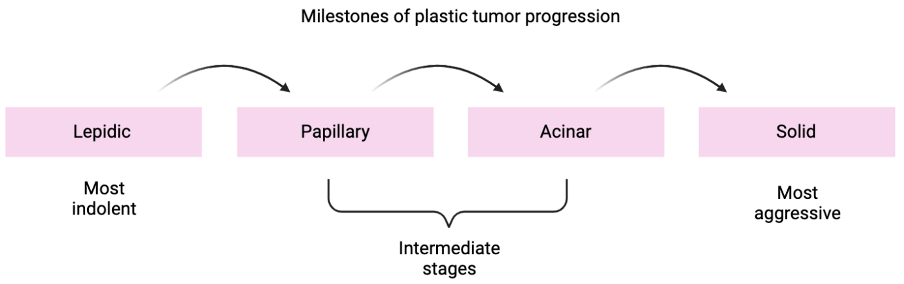
Whereas it is clinically relevant to identify the stages of tumor progression by histopathology, the molecular drivers of the transitions from one state to the next are also crucial. Towards this end, the team has characterized molecularly the transitions of cancer from lepidic to solid tumors, using a combination of techniques for single cell analysis of cancer cells and their interaction with the tumor microenvironment.
The team has the following aims: first is to detect molecular features of the transitions from lepidic to solid tumors across patients (see figure). Secondly, they will deepen our understanding of how tumor progression can be predicted based on the interaction of cancer cells with the tumor cell environment. The overarching goal of the project is to provide new insights into the role of cancer cell plasticity in disease progression and to explore if this helps predict disease progression in individual patients. Ultimately, the results will pave the way for new practices in clinical diagnostics as well as new approaches to lung cancer therapy.
Development of AI systems to assist in staging and treatment of bladder cancer patients

Bladder cancers (BLCA) are usually characterized as either superficial or invasive. As with all cancers, the tumor has to be staged in order to make a proper prognosis and decide on an appropriate course of treatment. Currently, bladder tumor staging depends on how deeply nested the tumor is into the bladder wall. However, recent research has shown that this may not reflect the most biologically relevant features of different forms of the disease. The aim of this collaboration is to better understand the biological behavior of bladder cancer on a molecular and cellular level in order to optimize the decision-making process for an effective course of treatment. This decision-making process is time-sensitive, as treatment must start by two weeks after the biopsy is taken.
There is a crucial need to optimize the staging process itself, as accuracy at this point will have a considerable impact on the choice of therapy and the patient’s quality of life. Both the over- and under-treatment of cancers can elicit serious problems.
The overarching goal of this TANDEM project is to explore the biological nature of BLCA through a range of advanced high-throughput molecular techniques (genomics, transcriptomics, epigenetics) combined with the monitoring of functional responses to different therapies. From this, an atlas of tumor cell types and sensitivities will be created, which will allow the researchers to generate an AI framework that should better guide BLCA patient care decisions. The collaborators bring different types of analysis together to shed light on both the tumor and its surrounding tissues (tumor microenvironment) and study the response of the BLCA to chemical inhibitors in vitro. The aim is to offer a more accurate platform for diagnosis and staging of BLCA, enabling clinicians to match patients with the most effective treatment.
Development of new therapy for patients with resistant colorectal cancer

Colorectal cancer (CRC) is the second most common cancer and is responsible for cancer-related deaths affecting men and women on a global scale. Even though therapeutic options have improved, including the promising introduction of immunotherapies, only a fraction of patients benefit or even respond to current treatments. For instance, checkpoint inhibitor therapy benefits only 5% of CRC patients so far. This collaboration aims to develop much needed novel therapeutic options, particularly ones adapted to the individual patient, in order to ensure higher efficacy and safety. Their goal is a better the quality of life and prognosis for CRC patients.
It has been shown that patients affected with CRC have an altered intestinal microbiota composition that may contribute to cancer pathogenesis, treatment resistance, and increased toxicity of currently used cancer treatments. Promising data now show that specific bacteria are able to modulate antitumor immune responses and may, therefore, serve as potential therapeutic agents or adjuvants for new therapies. Further new studies have established a link between higher levels of intratumoral eosinophils (a type of white blood cell with specialized immune function) and a favorable prognosis. The eosinophil levels correlate with enhanced survival of CRC patients.
The strategic aim of this project is to further the understanding of the patient’s microbiome and its interaction with the eosinophils to explore their potential roles as molecular biomarkers that could predict disease course and therapy response. Ultimately, this could lead to personalized microbiota-based precision medicine for CRC patients.
TCR-engineered CD4 T cell transfer for optimized cancer immunotherapy in adult and pediatric patients

This is a highly translational project aiming at harnessing the tumoricidal activities of CD4+ T cells to optimize cancer immunotherapies. The project includes preclinical validation of TCR-engineered CD4 T cells and the setup of a Phase 1 clinical trial for relapsed and refractory solid tumors in both adult and childhood cohorts.

